Computational Chemistry Projects at the University of Iowa in the Mason Group:
We have a number of active research projects, many involving collaboration with experimentalists. Feel free to contact Professor Mason to learn about other project areas or if you are interested in developing a collaboration!
VIDEO: See SEM talk about our research in the NSF Center for Sustainable Nanotechnology.

Center for Sustainable Nanotechnology
The Mason Group is a partner in this NSF Center for Chemical Innovation.
The center is based at the University of Wisconsin-Madison, with other partners at the University of Illinois, the University of Minnesota, Northwestern University, the Pacific Northwest National Laboratory, Wisconsin-Milwaukee, Tuskegee University, the University of Maryland-Baltimore County, Johns Hopkins University Augsburg College, and Georgia Tech.
Nanotechnology involves the use of materials at the smallest scale, including the manipulation of individual atoms and molecules. Products that use nanoscale materials (a nanometer is one-billionth the width of a human hair) range from beer bottles and car wax to solar cells and electric and hybrid car batteries. While there are hundreds of products that use nanomaterials in various ways, there are still lots of unknowns about how these modern materials and the tiny particles they are composed of interact with the environment and living things.
The center's overall goal is to minimize or eliminate the environmental impacts of nanoparticles while still keeping their technological advantages.
Our group will lead atomistic, quantum mechanical modeling and characterization of nanomaterials such as complex oxides used in battery materials and core-shell gold nanoparticles. The questions that Mason’s research will help the center address include delineating how nanoparticle structure dictates nanoparticle reactivity, what kinds of transformations nanomaterials undergo when their environment changes, and discovering the chemical pathways that lead to negative biological impacts of nanomaterials in the environment.
More about the CSN can be found at: http://susnano.chem.wisc.edu/
Aqueous Metal Nanoclusters
VIDEO: See Jennifer Bjorklund discuss Theory, Modeling, and Synthesis of Al Nanoclusters.
In collaboration with Prof. Tori Forbes, the Mason Group studies how nanocluster size, shape, and composition affects reactivity. We have used electronic structure calculations to discover "shape-reactivity" relationships in Al nanoclusters, noting that concave regions on the cluster surface gives rise to collective effects of neighboring water/hydroxyl functional groups, leading to strong gradients in electrostatic potential that can be related to adsorption trends. This tells us that it is not just the identity of functional groups, but their arrangement/distribution, that dictates how strongly molecules will interact with the cluster. This has implications for how contaminant species bind to nanoclusters, which relates to applications such as understanding geochemical reactions of environmental interfaces and water remediation strategies.

CAREER: Developing Quantum Nanogeochemistry for Molecular Studies and Inclusive Education
The Environmental Chemical Sciences Program in the Chemistry Division at the National Science Foundation supports the research of Professor Sara E. Mason at the University of Iowa who will direct a project whose ultimate goal is to develop quantum nanogeochemistry as a platform, (1) to provide fundamental understanding of environmental nanoparticle (ENP) structure-reactivity, (2) to merge distinct theories of ENP reactivity, and, (3) to recruit community college (CC) students for training opportunities at the university level. The research strategy is to carry out Density Functional Theory (DFT)-based simulations on two classes of ENPs: Aqueous aluminum hydroxides modeled as Giant Aluminum Polycations (GAPs) and mineral-water interfaces modeled as Periodic Slab Models (PSMs). Reactivity questions that are suited to each model category are identified, and systematic simulation experiments are designed using comparisons to isolate what controls reactivity. The projects are designed to develop atomistic simulations results of both GAPs and PSMs into new conceptual models for ENP reactivity, a goal that will support or dispel existing theories. For this purpose, the distinctions between the models will be exploited, for example, GAPs are ideal models for tracking reactivity with orbital interactions because they are truly nanoparticulate, and fewer electronic states are expected to participate in interfacial bonding.
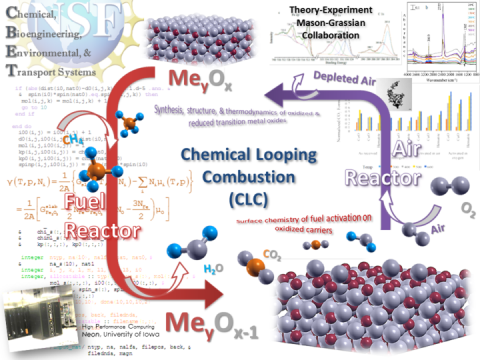
Insights into Chemical Looping Combustion Through a Combined Theoretical and Experimental Approach
Chemical looping combustion (CLC) is an emerging gaseous fuel combustion method in which carbon dioxide (CO2) can be separated from other components in the flue gas, enabling a low-cost and efficient separation and capture of CO2. The potential success of this project will help avoid negative impact on the earth's atmosphere and climate. The proposed activities will also promote training and learning activities by involving undergraduate and graduate students in research.
The research here addresses the need to develop a chemical understanding of two major factors governing metal oxygen carriers in CLC: The thermodynamic stability of the carrier after repeated redox cycling and mechanistic details of the heterogeneous reactivity. While batch studies have helped identify promising carriers, details of structure-reactivity relationships and the intermediate heterogeneous steps involved in the fuel oxidation are yet unknown. The proposed study aims to enhance the understanding of the fundamental reaction mechanisms involved in important heterogeneous processes of CLC and related catalytic applications. The thermodynamic stability of oxygen carriers will be assessed through experimental and theoretical methods and trends between carrier structure, composition, durability, and reactivity will be identified. The knowledge thus gained may drive the future rational design of materials for CLC and related applications. The methane fuel and its C-H bond activation related to many other reaction activities will be studied. Chemical synthesis and the replacement of petrochemical feed stocks by alkanes will also be investigated, expanding the potential impact of this project.

Collaborative Research: Interfacial Water Restructuring: An Unrecognized Contribution to Mineral Surface Reactivity
With this award, the Environmental Chemical Sciences Program of the Division of Chemistry is funding Professor Jeffrey G. Catalano of Washington University in St. Louis and Professor Sara E. Mason of the University of Iowa to investigate how the arrangement of water molecules near a mineral surface affects the adsorption of contaminants. Adsorption is a key chemical process at environmental interfaces that directly controls contaminant fate and nutrient availability and is an important precursor step in the nucleation and growth of precipitates, surface-catalyzed redox reactions, and microbial and ligand-promoted dissolution of oxide minerals. While adsorption mechanisms and the effect of surface potential on interfacial reactions are well established, little is known about the role of interfacial water. The information gleaned from these studies is expected to provide insight into fundamental reaction mechanisms involved in important environmental processes such as contaminant fate, transport, and degradation, nutrient bioavailability, carbon dioxide sequestration, and nanoparticle mobilization. This project integrates graduate students, undergraduate students, and high school student interns into the conduct of the research and supports new educational and outreach activities. A graduate student at Washington University will be trained in STEM pedagogies and then develop new active learning activities for a course on the environment and human health. At the University of Iowa, an annual museum exhibit will be prepared to educate K-12 students on the societal importance of geochemical surface science.
The objective of this project is to characterize adsorbate-induced interfacial water restructuring on mineral surfaces and its dependence on surface structure, surface charging, and fluid composition. Preliminary data shows that arsenate adsorption on aluminum oxide surfaces alters interfacial water structure. Wholesale restructuring occurred on a surface with weak initial water ordering whereas a small structural perturbation was observed on a surface with strong ordering. Such structural transitions of interfacial water are expected to affect the energetics and kinetics of interfacial reactions. This investigation integrates experimental and computational studies of interfacial water behavior on aluminum and iron oxide surface during arsenate adsorption. More specifically, the work integrates synchrotron-based surface crystallography techniques, laboratory measurements of adsorption isotherms and kinetics, density functional theory calculations, and ab initio molecular dynamics simulations to study how the structure of interfacial water responds to the adsorption of the contaminant arsenic and how such effects vary with mineral surface structure and charging. This study is anticipated to reveal a previously unrecognized mechanism that affects the energetics and kinetics of interfacial reactions.